Conformational Rheostats and Folding Coupled to Binding
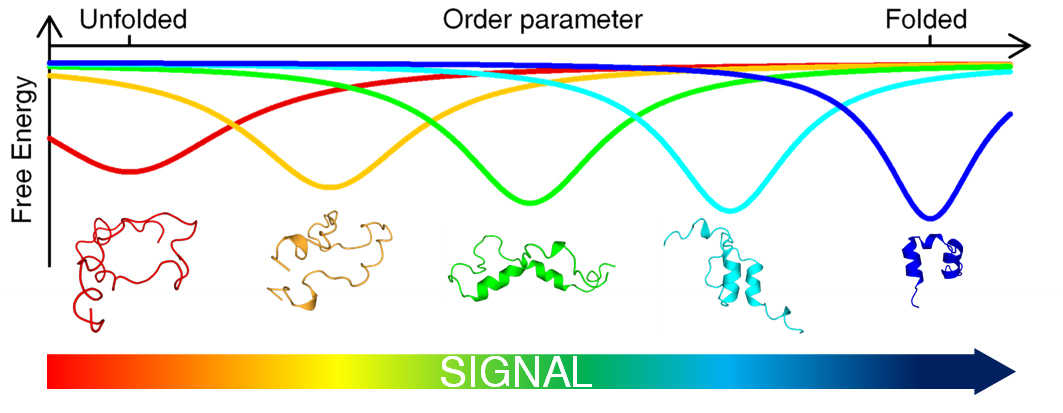
Extensive work on protein biophysics over the last two decades has unveiled a plethora of exceptions to the classical paradigm by which proteins fold into unique 3D structures in single strokes (two-state folding) and bind to their partners by simple structural complementarity (lock-and-key binding). We now know that a significant fraction of the proteome corresponds to proteins that are intrinsically disordered in their physiological stage. Intrinsically disordered proteins (IDPs) are able to fold upon binding in a process that may provide kinetic advantages and result in complex binding phenomena such as induced-fit and conformational selection. Some IDPs morph upon binding to structurally diverse partners, and others exhibit sophisticated allosteric behavior.
However, there is no quantitative understanding of the molecular mechanisms that lead to the striking conformational features of IDPs. The delicate interplay between folding and binding that must take place in these proteins, and the relationship between folding mechanism and complex binding behavior, remain unanswered questions as of today. There is even no mechanistic information on how the binding of an IDP to a partner influences its binding to other partners, or of how conformational disorder is related to allosteric binding behavior.